Does Carbon Capture Reduce Carbon Emissions?
Contributor: Frida Ruiz
Introduction
In order for carbon dioxide emissions to decrease enough to alleviate the effects of global warming and climate change, many scientists are pointing to carbon sequestration as a solution. Carbon sequestration is a method of trapping carbon dioxide preventing it from going into the atmosphere. Some sources claim that carbon sequestration has an 85-90% efficiency rate. Carbon dioxide can be captured physically or chemically. By Henry’s law, stating the amount of dissolved gas is proportional to the partial pressure of the substance dissolving, and carbon dioxide’s high partial pressures, this is made possible. Chemical absorption relies on carbon dioxide reacting with certain chemicals. Low temperatures and high pressures are used in order to efficiently execute both methods of carbon capture.
There are three main ways of capturing carbon dioxide: pre-combustion capture, post-combustion capture, and oxy-fuel.
Pre-combustion capture
In order to make the necessary mixture of hydrogen and carbon dioxide, fuel goes through gasification, a process where organic or fossil fuel get converted to carbon monoxide, hydrogen, and carbon dioxide. The process is also known as making low thermal gas into high thermal gas through steam deposition and pyrolysis.
Post-combustion capture
Carbon dioxide is captured by the exhaust of a combustion process. This is done by the following ways: absorbing carbon dioxide in a suitable solvent like monoethanolamine (MEA) and diglycolamine (DGA), a high pressure membrane filtration which rely on the permeability of carbon dioxide in order to capture it, adsorption/desorption process which binds carbon dioxide to a solid or liquid condense plate, or cryogenic separation which involves using low temperatures to cool certain gases in order to liquify carbon dioxide.
Oxy-fuel
Oxygen diluted with flue gas is used to combust the fuel resulting in a release of carbon dioxide and water. This then produces a stream of carbon dioxide making it easier to purify carbon dioxide.
Figure 1: This diagram explains the steps of each capturing method by stating how the raw materials are transformed.
Source: Carbon Capture and Storage, IPPC
After the capturing phase, the carbon dioxide gets transported by a pipe or ship. Then, the carbon dioxide is stored; essentially all storage works by storing the carbon dioxide captured into these deep formations where there are high temperatures and pressure. With the help of high temperature and pressure, the carbon dioxide would liquify. Carbon dioxide can be stored in former fossil fuel fields, deep saline formations, or depleting oil fields using one of these methods.
Structural storage
Injecting carbon dioxide deep underground specifically under a rock formation impermeable to carbon dioxide. Over time the carbon dioxide will build up underneath the rock and stay trapped.
Residual storage
Injecting carbon dioxide into a rock cap, and the small gaps between the rocks trap carbon dioxide.
Dissolution storage
Injecting carbon dioxide in a rock formation near an area of salty water. Over time carbon dioxide will go in the water, making the water more dense. This will make the water sink more, which traps carbon dioxide in the process.
Mineral storage
Carbon dioxide is chemically and irreversibly binding to the surrounding rock.
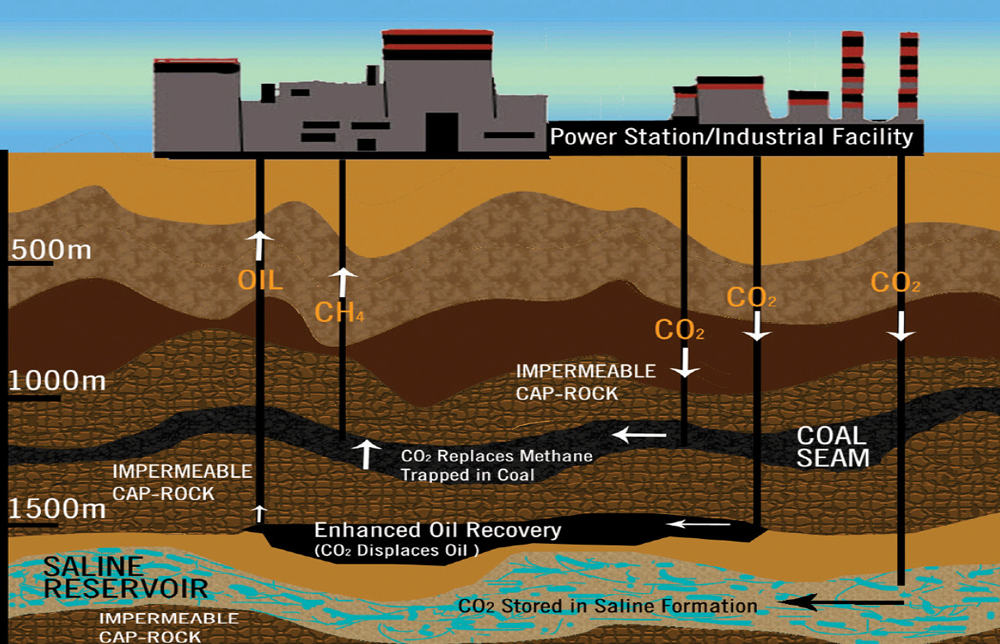
Figure 2: The captured CO2 is traveling down to be stored into the saline formation or under impermeable cap-rocks.
Source: CCS Diagram
The Problems/Greenwashing
Carbon capture has several misconceptions and flaws to the point where its validity is questioned by many scientists. Some have even claimed that carbon capture is an example of greenwashing- a market tactic which claims a product or process is good for the environment when that’s not the case.
First, it’s likely that some carbon dioxide will escape back into the atmosphere. Even a little bit of carbon dioxide escaping back into the atmosphere can cause a lot of damage. As stated by Gary Shaffer, a professor at the University of Copenhagen, “1% leakage every 10 years could mean a rise in 3-4 degrees in the next 2,000 years … 1% leaking every 100 years means the temperature would rise 2 degrees.” 1% leakage doesn’t seem like much, but carbon dioxide can stay in the atmosphere for hundreds of years. Even 1% can make a lasting effect.
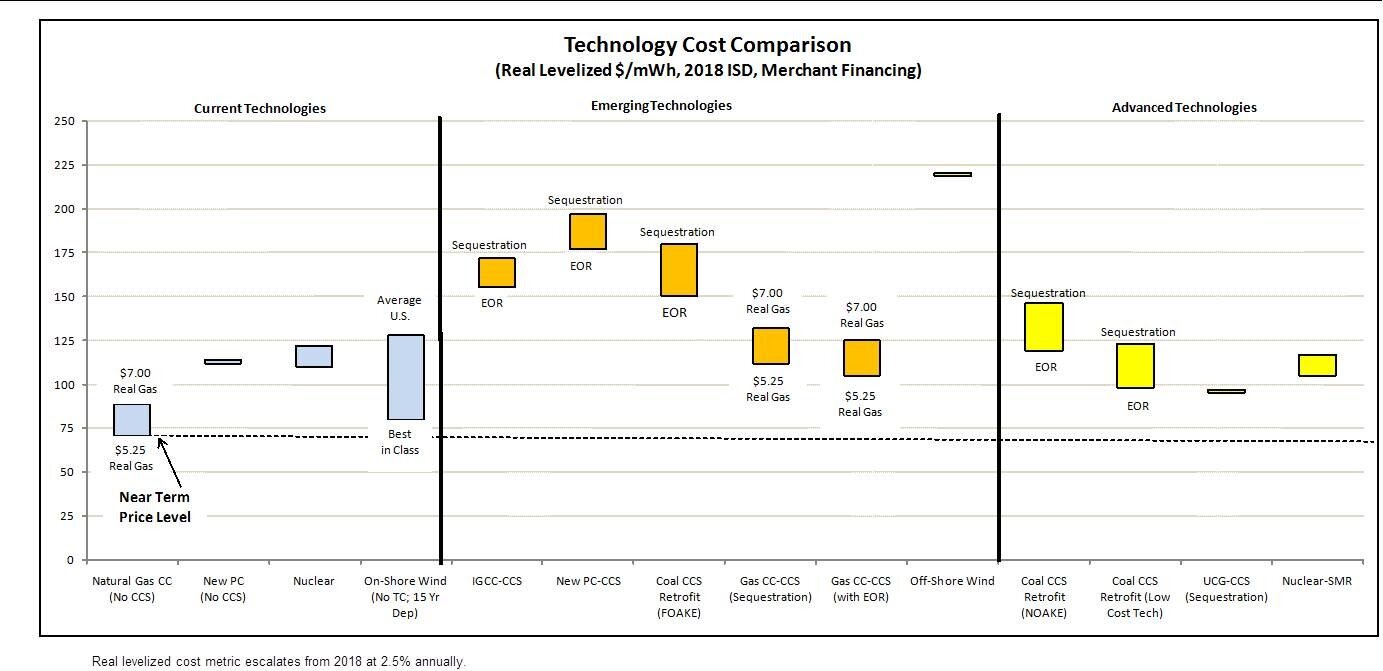
Next, the carbon capture is too expensive for the amount of efficiency it provides. This discourages companies from utilizing carbon capturing since they’re not necessarily gaining profit. The high cost is due to the resources needed to build carbon capture facilities. Not only are steel and concrete (two costly materials) required to make carbon capture, but in order to maintain the facility, electricity is needed. When compared to a plant that doesn’t implement carbon capture, the costs of electricity increase between 30 and 80%. Elecitiy is also used when compressing CO2 to make it ready for transport and injection.
Figure 3: A diagram showing that technologies like carbon capture are more costly than current technologies.
Source: Costs and Challenges of Carbon Capture and Storage ,Clean Air Task Force
Third, carbon sequestration can negatively affect the geology and the land around the storage areas. The groundwater around these storage areas has been said to be more acidic and have an increased concentration of iron, cadmium, and cobalt zinc. This has led to water which is unsafe to drink. According to researchers at Duke University, there is a high risk of captured carbon leaking into water aquifers therefore making the water undrinkable.
These flaws are why many environmental organizations, such as the Sierra Club disregards carbon capture as a viable option. Other opponents claim that in order to make any viable change, there will have to be many carbon capturing projects which is unrealistic due to its cost. It’s better to invest that money in renewable energy, and, without any real change, environmentalists will continue to consider carbon capturing as an example of greenwashing, thus giving oil and coal companies an excuse to continue with what they’re doing.
New Innovations
Finding ways to improve carbon capture technology is crucial in order to ensure this could be a legitimate way to reduce carbon dioxide emissions. There are new innovations that will not only increase the efficiency of carbon capture, but also lower costs.
One change would be to implement solid adsorbents instead of using water as an absorbent. One such absorbent was developed at Georgia Tech used polyethylenimine-silicon dioxide (PEI) in order to make solid hollows; then flue gas passes through these solid hollows. These solid hollows are able to absorb carbon dioxide just as efficiently as liquid absorbents. In order to release the carbon dioxide from the fibers to store it, scientists run the fibers under hot water. Another useful innovation was made by Jeffrey Long at UC Berkeley : metal–organic frameworks (MOFs). MOFs are made from magnesium and diamine molecules (two amino groups) loaded into the pores; they have a large internal surface area making them a good material to absorb carbon dioxide. MOFs are able to absorb carbon dioxide by ensuring it binds to a diamine in a way that encourages more carbon dioxide molecules to bind to the neighboring diamine molecule. High temperatures are used to release the carbon dioxide from the fibers.
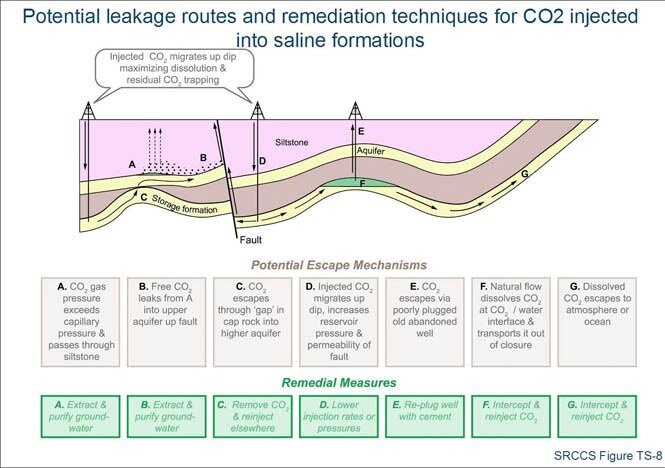
Finally, to improve storage methods so carbon dioxide won’t have the chance to escape back into the atmosphere. Saline water reservoirs are very effective in keeping carbon dioxide stored. This is because carbon dioxide lays under an impermeable layer of shale and the sandstone pores lock carbon dioxide in place. Over time, carbon dioxide reacts with brine to solidify into calcium carbonate. An example of this put into action is the Statoil natural gas plant which has used this method to store over 1 million metric tons of carbon dioxide without any leakage. In addition to the efficiency of the reservoirs, there are enough reservoirs available to store 2,600 billion metric tons of carbon dioxide.
Figure 4: Describes how saline formations could block off leakage routes.
Source: The Problem With Carbon Capture: CO2 Doesn’t Always Stay Captured, Fast Company
Conclusion
Carbon Sequestration is a solution that needs more research before expanding the implementation around the world. It’s crucial to ensure that the process isn’t going to do more harm than good. Carbon capturing should only be used shorterm, and should be geared to steel and cement manufacturing since it’s hard to reduce CO2 due to the amount of steel and cement needed. Student groups are able to help by educating others about what carbon capture is and some of the misconceptions. Additionally, student groups can call companies asking them how they are ensuring they emit less CO2. By clarifying misconceptions and building upon the current research, we could strive to bring CO2 back into the ground where it’s wanted instead of the atmosphere where it’s exacerbating the effect of climate change.